CLINICAL SOLUTIONS
CLINICAL SOLUTIONS
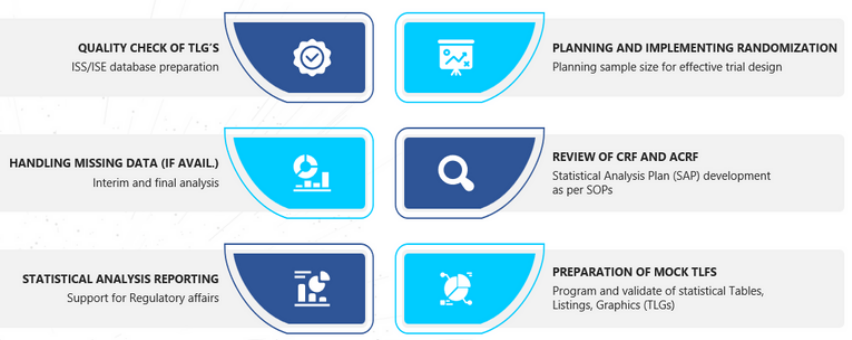
CLINICAL ANALYTICS & DATA MANAGEMENT
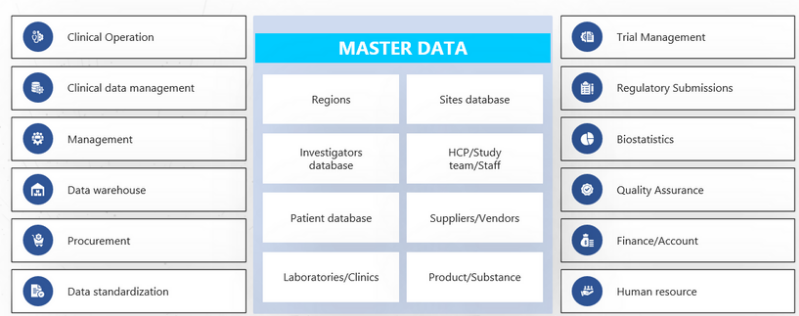

REAL WORLD DATA
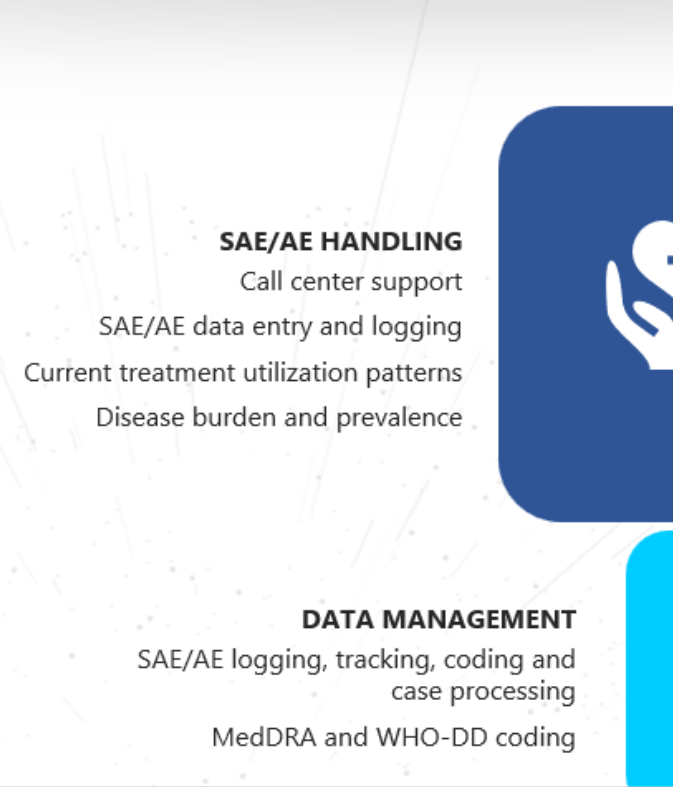
PHARMACOVIGILANCE
post-market product safety efforts.
With a worldwide footprint and a foundation rooted in quality systems, we harness advanced technology to handle and report adverse events (AE) and serious
adverse events (SAE). Our expertise spans diverse domains, resulting in a holistic safety knowledge base that delivers tangible benefits to pharmaceutical,
biotechnology, and medical device companies.
REGULATORY
AKT Health stands as your unwavering partner throughout your regulatory journey. With a dedicated team, precise strategies, and compliance expertise, we bring your products to market, ensuring both regulatory approval and long-term success.
Our tailored strategies guide your product through global approvals.From submission management to electronic publishing, we ensure compliance across IND, NDA, BLA, and CTA applications. Our in-house experts handle compliance, interim management, and post-marketing surveillance
Medical Writing

Connect with our Expert


